Delivering a Wide Range of Virtual and Hybrid Approaches to Decentralized Clinical Trials
We offer
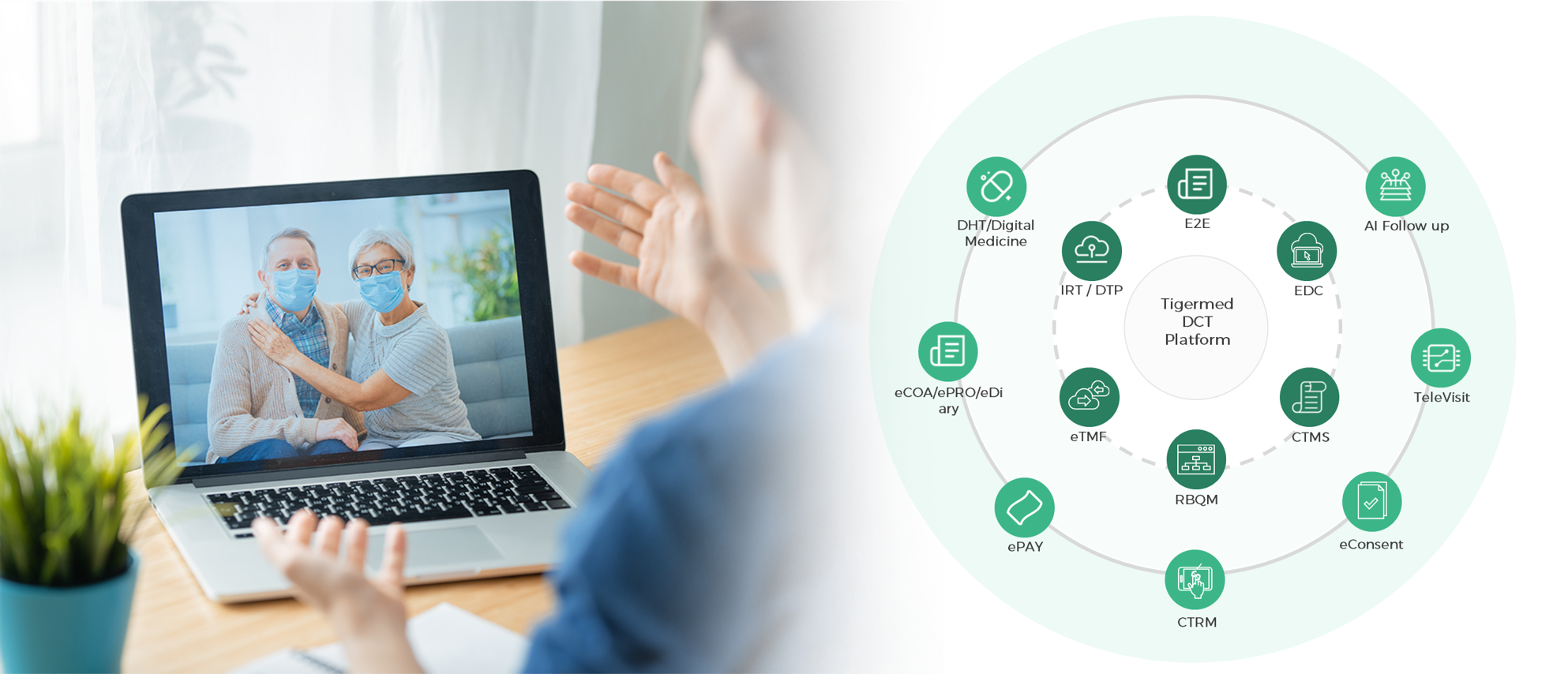
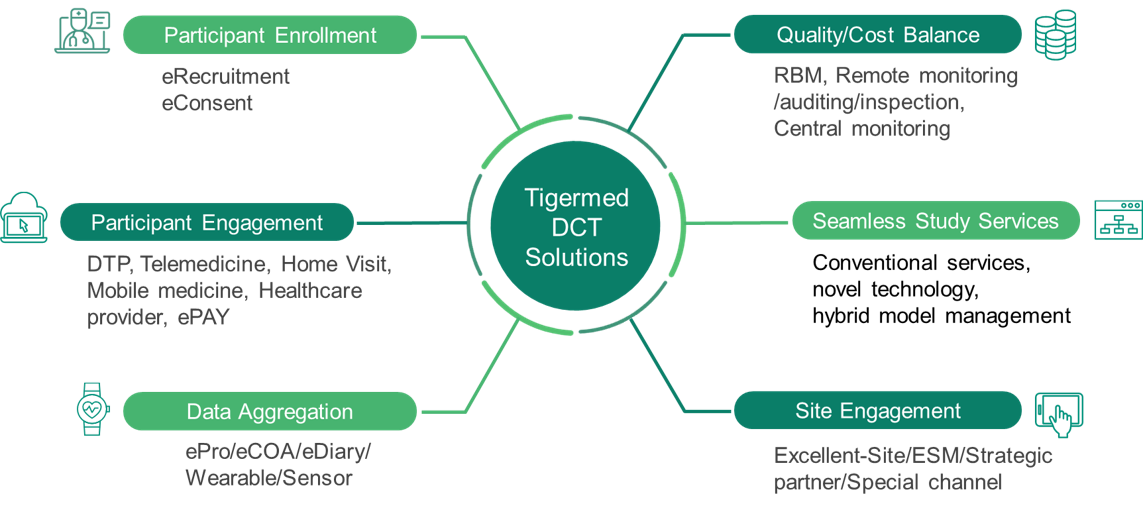
flexible solutions for digital and decentralized clinical development. Our DCT
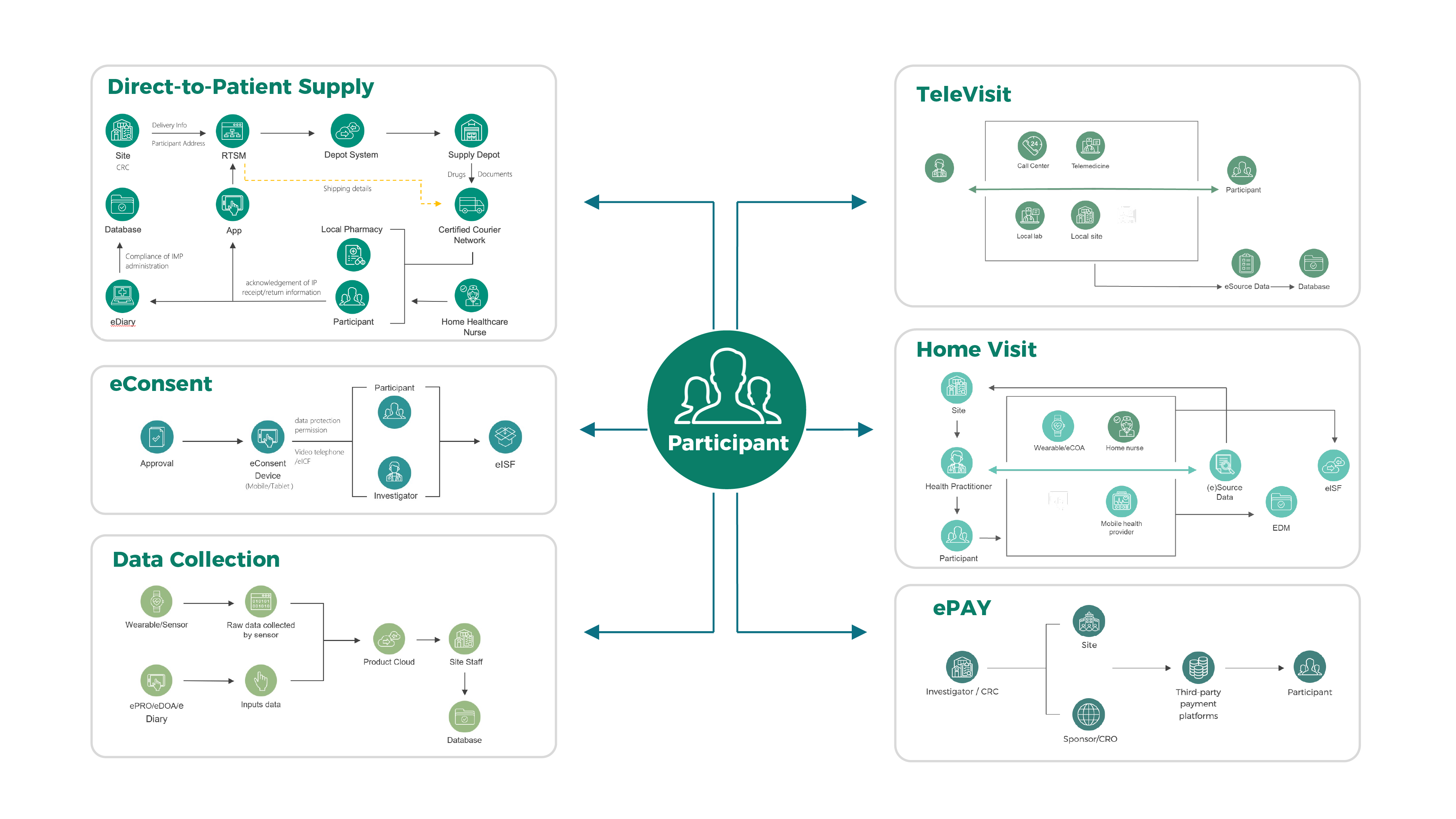
solutions leverage in-house expertise, including eConsent, TeleVisit,
Direct-to-Patient Supply, etc., as well as cutting-edge technologies such as RBQM,
eCPM, and ePay. These comprehensive solutions guide you through the planning, operation,
and delivery of decentralized clinical trials. Our experience includes over
200+ clinical trials using a DCT hybrid model, spanning the full research
spectrum of indications, including oncology, vaccines, infectious diseases, and
more.