Comprehensive and Agile Medical Writing Services
We provide tailored and high-quality medical writing expertise across a broad range of therapeutic areas and document types, from IND application to clinical trial to post-marketing writing. With more than 20 years of experience partnering with pharmaceutical, biotech, and medical device clients, we offer protocol design and medical writing services in oncology, hematology, orthopedics, cardiovascular disease, respiratory, infectious disease, vaccine, etc., and dive into delivering high-quality solutions to meet full regulatory compliance.
- In-house medical writers: 85% with M.S. degree, 20% with PhD. degree
- Over 20 years of experience partnering with pharma, biotech and clinical site to provide customized medical writing solutions
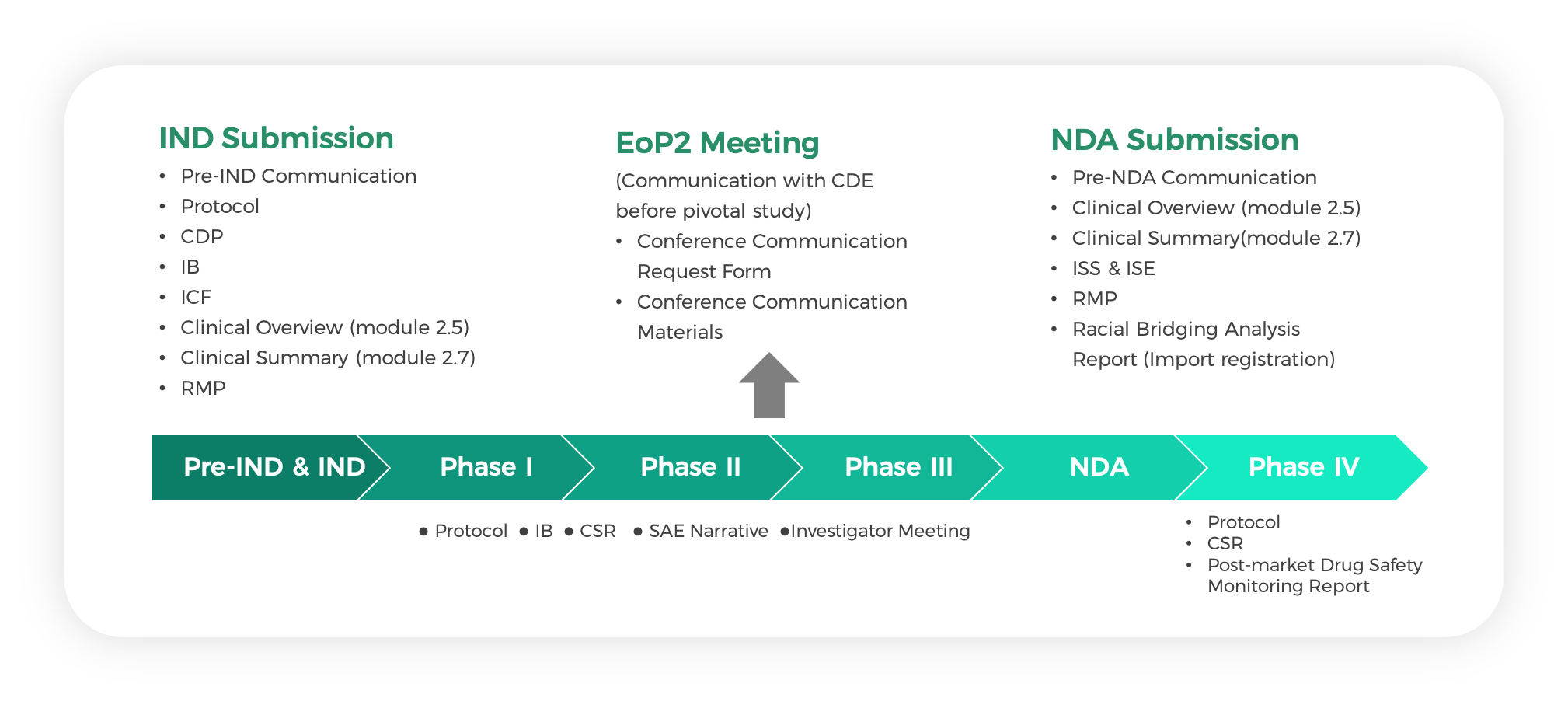
- A complete writing service spanning from individual documents to extensive writing programs, including: protocols, IND applications and NDA submission documents, informed consent forms, clinical development plan, clinical study reports, integrated summaries of safety and efficacy, etc.
- Work closely with Biostatistics, Regulatory and Medical experts, and operational team to access a fully integrated network of information