30+
Non-Covid vaccine clinical studies
150K
Enrolled subjects in global vaccine studies
70+
Approved vaccines we served
Our Company
Culture & Values
Leadership
Global Locations
Corporate Compliance
Subsidiaries & Joint Ventures
Diversity & Inclusion
20th Anniversary
Tigermed EMEA
Medicinal Chemistry
Compound Screening
DMPK
Safety & Toxicology
Bioanalytical
CMC
Medical Writing
Clinical Monitoring
Regulatory Affairs
Data Management and Statistical Analysis
Clinical Development Strategy
Decentralized Clinical Trials (DCTs)
Site Management (SMO)
Medical Device / IVD
Multi-region Clinical Trial (MRCT)
Vaccine Clinical Trial

Clinical Development
Vaccine Clinical Trial
A combination of therapeutic depth and global capabilities make us a valuable partner for vaccine research.
Your Vaccine Clinical Trials Start Here
Vaccine research is unique in its ability to have widespread impact on global health. Now more than ever, vaccine development requires a more innovative and efficient approach. Our vaccine service scope covers vaccine clinical design, monitoring, data statistics and biological testing, we work closely with you to develop personalized roadmaps for your vaccine study and aims to maximize efficiency, anticipate challenges, and mitigate risk.
Non-Covid vaccine clinical studies
Enrolled subjects in global vaccine studies
Approved vaccines we served
Covid-19 clinical studies
Percentage of Phase III studies
Provincial CDCs in collaboration

A Broad Range of Expertise
S. aureus
Zoster
Covid-19
Therapeutic BCG
Influenza
Malaria
Rabies
Nasopharyngeal Cancer
Hepatitis B
Poliomyelitis
Rotavirus
Live Attenuated Varicella
ECM
HIV
HPV
Mumps
Vaccine Clinical Research Process
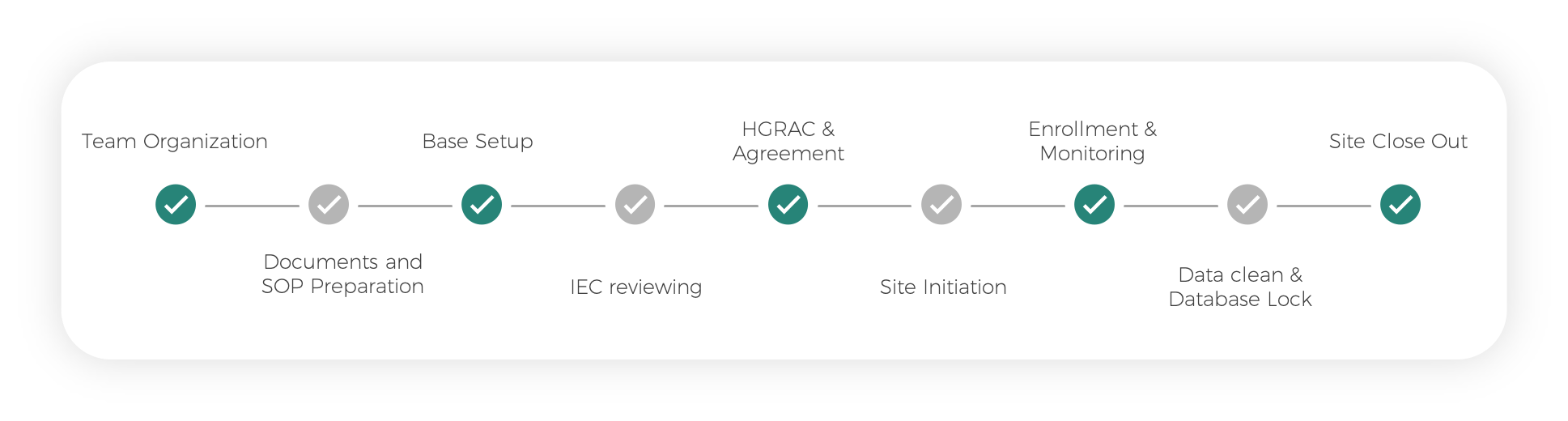
Vaccine Clinical Research Process
Team Organization
Documents and SOP Preparation
Base Setup
IEC reviewing
HGRAC & Agreement
Site Initiation
Enrollment & Monitoring
Data clean & Database Lock
Site Close Out
A Unique Partner for Vaccine Clinical Trials
Case Study
From late 2020 to early 2021, Tigermed teamed up with CanSinoBio and Pakistan local expert, as well as clinical professionals from Mexico, Russia, Chile, Argentina, to conduct Phase III trial of COVID-19 vaccine and ultimately delivered the study. This is also the first China-initiated phase III vaccine clinical study covering multiple continents, including Asia, Europe, and Latin America.
The site uses cookies in order to collect data to provide general statistics to optimize site functionality and offer you a better experience. For more information, visit our Privacy Policy.